Class 9th Chemistry Unit 4: Organic Chemistry Notes
Organic chemistry is an integral part of the Class 9th chemistry syllabus, providing a foundation for understanding the chemistry of life. Unit 4 of Class 9th Chemistry delves into organic chemistry, covering essential topics like hydrocarbons, functional groups, and various organic compounds.
This article will provide an in-depth look into Unit 4 of Class 9th chemistry, explaining key concepts while offering valuable study tips to help students ace their exams.
We will also include an link to additional educational resources and materials available at Sufa Stationers.
What is Organic Chemistry?
Organic chemistry is the branch of chemistry that deals with the study of carbon-containing compounds. It primarily focuses on hydrocarbons (compounds made of hydrogen and carbon) and their derivatives. Organic chemistry plays a crucial role in understanding many natural and synthetic processes, from biological systems to industrial applications.
In Class 9th Unit 4, students are introduced to basic organic compounds, their classification, and their significance in everyday life.
Key Topics in Class 9th Unit 4: Organic Chemistry
1. Introduction to Organic Compounds
Organic compounds are compounds that contain carbon atoms. They are the basis of all living things and can also be found in many synthetic materials. In this chapter, students will learn about the importance of carbon in forming a variety of compounds due to its unique ability to bond with itself and other elements.
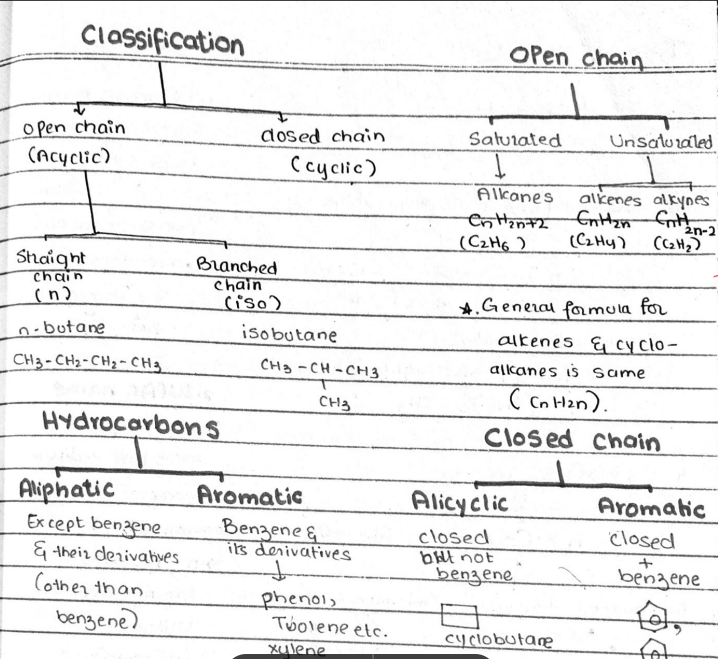
2. Classification of Organic Compounds
Organic compounds are classified into several categories based on their structure and composition:
- Hydrocarbons: These are compounds that only contain hydrogen and carbon. They are further classified into:
- Alkanes (Saturated hydrocarbons)
- Alkenes (Unsaturated hydrocarbons with double bonds)
- Alkynes (Unsaturated hydrocarbons with triple bonds)
- Functional Groups: A functional group is a specific group of atoms that are responsible for the characteristic chemical reactions of a compound. For example, alcohols contain a hydroxyl group (-OH), and carboxylic acids contain a carboxyl group (-COOH).
3. Nomenclature of Organic Compounds
Understanding how to name organic compounds is a critical skill in organic chemistry. In Class 9th Unit 4, students will learn the basics of the IUPAC system for naming organic compounds, including the naming of alkanes, alkenes, and alkynes.
For example:
- Methane (CH₄) is the simplest alkane.
- Ethene (C₂H₄) is an example of an alkene.
- Ethyne (C₂H₂) is an example of an alkyne.
Class 9th Chemistry Unit 4 Notes
chapter-no-4Important Concepts in Class 9th Organic Chemistry
1. Saturated and Unsaturated Hydrocarbons
- Saturated hydrocarbons (Alkanes) have single bonds between carbon atoms. They are generally less reactive.
- Unsaturated hydrocarbons (Alkenes and Alkynes) have one or more double or triple bonds between carbon atoms, making them more reactive than alkanes.
2. Functional Groups and Their Importance
Functional groups are vital in determining the properties and reactions of organic compounds. For example:
- Alcohols contain the hydroxyl (-OH) group and are used in making disinfectants, solvents, and perfumes.
- Carboxylic acids contain the carboxyl (-COOH) group and are found in substances like vinegar and other food preservatives.
By understanding these functional groups, students can predict how different organic compounds will react under various conditions.
3. Isomerism
Isomerism is the phenomenon where two or more compounds have the same molecular formula but different structural arrangements. The study of structural isomers in Class 9th Unit 4 introduces students to how compounds can have varying physical and chemical properties despite having the same number of atoms.
4. Polymerization
Polymerization is a chemical reaction in which small molecules (monomers) combine to form large chains (polymers). Common examples of polymers include plastics, rubber, and synthetic fibers. This section covers how organic compounds like ethylene undergo polymerization to form polyethylene, a widely used plastic.
Practical Applications of Organic Chemistry
Organic chemistry plays a significant role in various industries, from pharmaceuticals to petrochemicals. Some real-world applications that students will encounter in Class 9th chemistry notes include:
- Fuels: Hydrocarbons like methane, ethane, and propane are used as fuels in vehicles and homes.
- Plastics: Many household items, such as plastic containers and bottles, are made from polymers.
- Medicine: Organic compounds are essential in creating medicines, including antibiotics and pain relievers.
Tips for Studying Class 9th Unit 4 Organic Chemistry
1. Understand the Basics
Organic chemistry is all about understanding how carbon compounds interact. Focus on grasping the basics of bonding, molecular structures, and functional groups before diving into complex reactions.
2. Practice Naming Compounds
Learning the IUPAC system for naming compounds can be tricky at first. Practice naming various hydrocarbons, alcohols, and acids until you’re confident. Try writing down formulas and naming them to reinforce your learning.
3. Memorize Key Reactions
Memorizing reactions involving alkanes, alkenes, alkynes, and functional groups is essential for scoring well in exams. Flashcards can be a helpful tool for this.
4. Utilize Diagrams and Charts
Organic chemistry often involves visualizing molecules and reactions. Diagrams of structural formulas and reaction mechanisms can help you better understand how organic compounds interact.
For more resources like flashcards, solved papers, and additional study materials, visit Sufa Stationers for all your educational needs.
Conclusion
Unit 4 of Class 9th Chemistry introduces students to the fascinating world of organic chemistry. From learning about hydrocarbons and functional groups to understanding key reactions and applications, this unit lays the groundwork for more advanced topics in the future. By using well-organized notes, practicing the nomenclature, and memorizing reactions, students can excel in their exams.
For more detailed Class 9th chemistry notes and educational resources, be sure to check out Sufa Stationers. We offer a wide range of study materials, including solved past papers, notebooks, and other educational resources to help you succeed in your academic journey.